Your patients with cachexia and metastatic pancreatic ductal adenocarcinoma, able and planning to receive first-line chemotherapy, could be eligible. Find out more and take the first steps to refer your patients, today.
Patients with cancer often have elevated levels of the cytokine GDF-15, which is associated with cachexia and reduced quality of life. Ponsegromab is an anti-GDF-15 therapy, which may improve metabolic and immune-mediated change in the tumor microenvironment. The RIVER Pancreatic trial aims to investigate the efficacy, safety, and tolerability of ponsegromab, versus placebo, on body weight, appetite, physical activity and overall survival, building on previous Phase 1b and Phase 2 studies.
Who may participate
RIVER Pancreatic is enrolling adults (18+) who are:*
- diagnosed with histologic or cytologic active metastatic pancreatic ductal adenocarcinoma (locally advanced disease is not eligible);
- experiencing cancer cachexia (as per the Fearon criteria),†
- able and planning to receive Cycle 1 of first-line, systemic chemotherapy (nab-paclitaxel and gemcitabine or FOLFIRINOX) at the time of the screening visit;
- and have an ECOG PS ≤1 and life expectancy of ≥4 months.
Participants must not have:*
- prior radiotherapy, surgery, chemotherapy,‡ or investigational therapy for metastatic disease;
- a history of heart failure;
- current active reversible causes of decreased food intake;
- or tube feeding or parenteral nutrition treatment
Importantly, patients must be screened before they start first-line chemotherapy.
*A full list of eligibility criteria will be available to you in the study protocol.
†Involuntary weight loss of >5% within 6 months prior to screening irrespective of BMI, or BMI <20 kg/m2 and involuntary weight loss of >2% within 6 months prior to screening
‡Unless adjuvant setting chemotherapy, with at least 6 months since completion of the last dose, and no treatment-related toxicities.

Key objectives
To evaluate the effect of ponsegromab compared with placebo on body weight.
To evaluate the effect of ponsegromab compared with placebo on the appetite-related symptoms as measured by Functional Assessment of Anorexia/Cachexia Therapy 5-item Anorexia Symptom Scale (FAACT 5IASS).
What to expect

Screening period
- Screening visit will occur no more than 14 days prior to Cycle 1 Day 1 of first-line chemotherapy treatment.
- All consented participants should undergo all screening procedures prior to starting first-line chemotherapy for metastatic disease.
First-line chemotherapy
- All participants will receive first-line, systemic chemotherapy before randomization (double-blind treatment period Day 1). This will be a PI choice of either:
- 1 × 28-day cycle of nab-paclitaxel and gemcitabine chemotherapy, or
- 2 × 14-day cycles of FOLFIRINOX chemotherapy (mFOLFIRINOX is acceptable).
- All chemotherapy dosing is to be determined by the participant’s health care provider in accordance with local guidelines.
- Patients must not have received chemotherapy treatment for metastatic disease in the previous 6 months before screening.

Double-blind period (Phase 2b)
Enrollment will begin after completion of the 1 to 2 cycles of first-line chemotherapy.
Study treatment
- Participants will continue on first-line chemotherapy
- Participants will be randomized 1:1 to receive 1 of 2 doses of the study medicine or placebo
- After Dose Selection analysis, participants taking the non-selected dose of the study medicine will be switched to the selected dose. Participants on placebo will continue on placebo
- Once Phase 3 is completed, participants will have the opportunity to enter the open-label extension period

Double-blind period (Phase 3)
Enrollment will begin once phase 2b enrollment is complete.
Study treatment
- Participants will continue on first-line chemotherapy
- If enrolled before Dose Selection: Participants will be randomized 1:1:1 to receive 1 of 2 doses of the study medicine or placebo
- After Dose Selection, participants randomized to the study medicine will be switched to the selected dose. Participants on placebo will continue on placebo.
- If enrolled after Dose Selection: Participants will be randomized 1:1 to receive the study medicine or placebo
- Once the study is completed, participants will have the opportunity to enter the open-label extension period (see below)

Open-label extension (OLE) period
- Participants will continue on first-line chemotherapy
- Phase 2b and Phase 3 end when approximately 517 overall survival events are accrued in during the Phase 3
- All active participants (Phase 2b and Phase 3) will be offered the choice to enter the OLE. Participants on the selected dose will continue on that dose. Participants on placebo will be switched to the selected dose
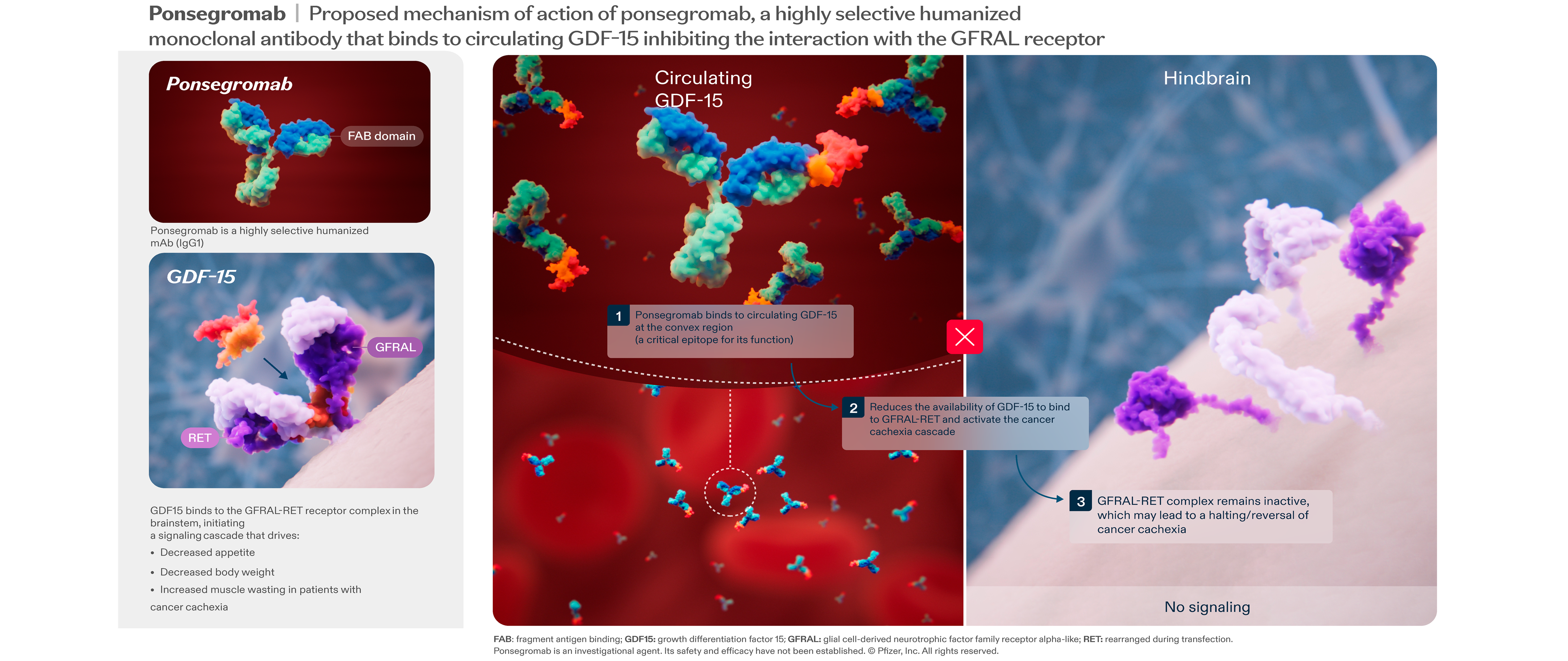
About Cachexia, GDF-15 and ponsegromab
Cancer cachexia is a complex, multi-organ syndrome caused by upregulation of systemic inflammatory stimuli leading to an imbalance in metabolism. People with cancer have higher serum levels of growth differentiation factor 15 (GDF-15), which is secreted by tumor cells, macrophages and damaged cells. Elevated GDF-15 is associated with cachexia symptoms, as well as poor outcomes and reduced survival.
Ponsegromab is an anti-GDF-15 therapy that may be able to help improve symptoms of cancer cachexia. Anti-GDF-15 therapy is designed to act through a combination of metabolic improvement and immune mediated change in the tumor microenvironment. Thus, ponsegromab has the potential to improve tolerance, adherence and efficacy of chemotherapeutic regimen(s) and contribute to prolonged survival.
For more details on previous data presented, visit: Pfizer Presents Positive Data from Phase 2 Study of Ponsegromab in Patients with Cancer Cachexia | Pfizer
Get started
Answer a 2-minute questionnaire and speak to a study representative.
A first step as you consider connecting with a Principal Investigator is to answer a 2-minute online questionnaire about your interest and willingness to be contacted. If your answers show the study might be a good fit for you and your patient, you may choose to have your contact information shared with a study clinic that you select for further discussion.
Get connected.
Your answers to these questions will only be linked to you if your responses indicate that you would like to be connected with a Principal Investigator and you choose to share your contact information with the study clinic. Pfizer study team members and our partners will have access to reports containing aggregated data that will not be directly linked back to you. Only the study staff can determine if your patient meets the study’s eligibility criteria and is able to enroll in the study.
Cachexia is a complex, multi-organ syndrome caused by upregulation of systemic inflammatory stimuli leading to an imbalance in protein and muscle metabolism. People with cancer have higher serum levels of growth differentiation factor 15 (GDF-15), which is secreted by tumor cells, macrophages and damaged cells. Elevated GDF-15 is associated with cachexia symptoms including body weight loss, as well as poor outcomes and reduced survival.
Eligible participants are those experiencing cancer cachexia as defined by either:
- The Fearon criteria: BMI <20 kg/m2 and involuntary weight loss of >2% within 6 months prior to screening or
- Involuntary weight loss of >5% over the past 6 months prior to screening, irrespective of BMI
To date, there are no approved medicines in the US and 1 medicine approved in Japan (anamorelin) to treat cancer cachexia. Other medications (e.g., dronabinol, glucocorticoids, megestrol, metoclopramide, and olanzapine) are often used off-label.
In previous Phase 1b and Phase 2 studies, gain in body weight and reduction of cachexia symptoms (including appetite, fatigue, and impaired physical function) were observed. Find out more here: Pfizer Presents Positive Data from Phase 2 Study of Ponsegromab in Patients with Cancer Cachexia | Pfizer.
Ponsegromab (or placebo) will be administered in a divided dose requiring 2 subcutaneous injections, given in rapid succession, every 4 weeks. Permitted injection sites are the abdomen, upper arm, and thigh, which can be rotated with each administration for participant comfort.
The double-blind period is expected to last for approximately 1 year, with around 14 study visits during that time.
If the study is deemed not complete by Week 48, the participant will continue on an every 1-month visit schedule and the list of procedures to be performed will continue as for Week 40 to Week 48 with the same regularity and the same visit window until study completion (e.g., 52, 56, 60 weeks etc.) Unscheduled visits are allowed at any time as needed for assessment of AEs, repeat laboratory testing, etc.
When the number of overall survival events has been accrued to terminate the study, participants will then either 1) complete an end of study visit or 2) enter the optional open-label extension.
Following enrollment completion of Phase 2b, Phase 3 enrollment will begin. One of the two ponsegromab doses will be selected once all Phase 2b participants have completed Week 12 procedures. Eligible Phase 3 participants enrolled after dose selection will be randomized 1:1 (ponsegromab selected dose, or placebo). Depending on which study intervention they were initially enrolled upon, all continuing participants will either remain on the selected dose in Phase 3, or be switched to the selected dose, or continue receiving placebo. All participants will remain blinded to the study intervention until the end of Phase 3.
Eligible participants will have the option to enter the open-label extension, which is planned to last for a further 48 weeks. Similar study assessments will take place over up to 14 study visits, including regular physical health assessments, adverse event monitoring, and clinical laboratory tests. Ponsegromab will be administered every 4 weeks.
Potential participants may be directed to www.river1021.com to learn more.
Further information about the RIVER Pancreatic trial can be found using the following details:
Protocol Number: C3651021
EU CT Number: 2025-522093-36-00
ClinicalTrials.gov ID: NCT06989437
- Regular assessments of body weight, vital signs, pregnancy status, adverse events.
- Physical examinations, 6-minute walk tests, physical function questionnaires, and monitoring of physical activity via wrist-worn activity watch.
- CT scan (or MRI) for tumor imaging and body composition assessment approximately every 6–8 weeks.
- Clinical laboratory tests including: hematology, blood chemistry, key biomarkers [China: ,and HBV, HCV, HIV, and syphilis serology].