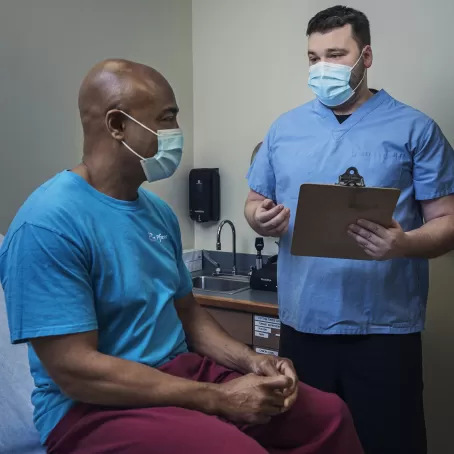
The bulk of any trial at the PCRU is carried out in the clinic, where staff perform dozens of procedures throughout any given day and have high levels of contact with participants. Among these staff, the Clinic Coordinator serves to streamline the trial’s conduct. Winston Halstead describes the Clinical Coordinator’s role as a sort of “universal hub” between the different teams at the PCRU.
“During a study, the Clinical Coordinator is really a foot soldier on the ground,” he says. “We’re talking to everyone - the Principal Investigator, the lab, the pharmacists, the clinic staff, the participants, every part of the PCRU. Basically, we’re there to make sure the study is being executed according to the protocol.”
The job, Winston says, is, “very hands on,” and starts when a PCRU study participant first walks in the door.
“I’m the first person you’ll see when you check in for a study,” Winston says. “It’s a Clinical Coordinator who greets and guides you. We also educate everyone about the study and what to expect, answer any questions.”
And as the study proceeds, the Clinical Coordinator is in constant contact with those participants. Winston helps help explain the importance of the rules and requirements, both for the study and the PCRU as a whole.
“COVID has affected everybody,” Winston explains. “So, working on something that might actually help everyone - that’s really exciting.”
“We want to keep you safe,” he tells volunteers. “The rules are designed a certain way for that reason, and everything we do is scrutinized and studied carefully. We have an eye on everything, in terms of their health and safety.”
In the three years since Winston joined the PCRU, he’s worked on all sorts of clinical trials, designed to study all sorts of potential medicines.
“But the studies we did during the pandemic felt a little different,” Winston says. It was his work with participants in a groundbreaking COVID-19 oral treatment trial that felt uniquely critical.
“COVID has affected everybody,” Winston explains. “So, working on something that might actually help everyone - that’s really exciting.”
Winston first came to the PCRU as a side job, collecting data and working extra hours to pay for nursing school. That experience provided Winston with his first exposure to the world of clinical research.
“It wasn’t really something you learned about in nursing school,” he says. But during his months working in at the PCRU Winston got a sense of how clinical research works and discovered his personal interest in improving it.
In the midst of the pandemic, Winston made the switch to a full-time staff position at the PCRU, completed his continuing education coursework, and took on the role of Clinical Coordinator, eventually working his way up to be Clinic Manager.
Along the way, he found that working on a study dedicated to helping address the COVID-19 Pandemic challenged and motivated him to excel in his professional role.
And like his colleagues on the PCRU team, Winston also discovered that his personal dedication to the trial was mirrored and heightened by the commitment of the trial participants themselves.
“That’s true for me, our team, and for the participants in the studies too, absolutely,” Winston says. “It makes you feel like you're making a difference.”