Lung cancer can affect anyone — regardless of age, race, ethnicity, health history, or smoking status. Despite recent advancements, clinical trials remain essential for continuing to improve and refine lung cancer treatments.
Clinical trials help researchers learn how safe and effective potential medical treatments are when given to participants. All medicines that become available today are first tested in hundreds to thousands of participants. Clinical trials are essential for modern medicine to advance. When joining a clinical trial, you play an important role in that progress.
The decision to join a clinical trial is personal, and it is yours. Participating in a clinical trial has the potential to impact you as well as many others affected by the same or similar medical condition.

Common eligibility criteria in lung cancer clinical trials
Every clinical trial has requirements to participate, called eligibility criteria. They may include:
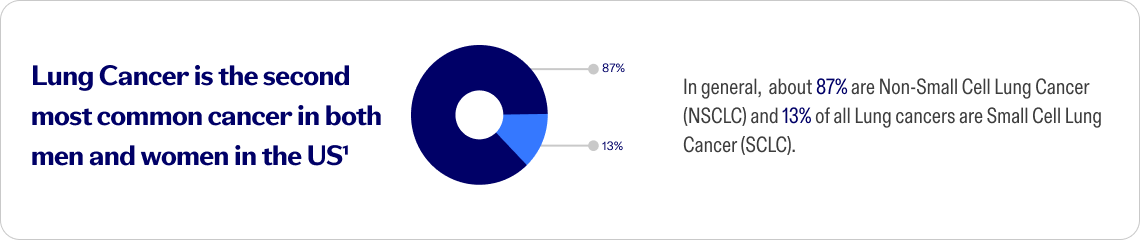
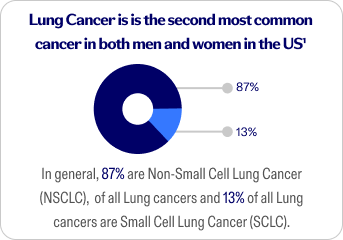
Non-Small Cell Lung Cancer (NSCLC)
The most common type of lung cancer. It happens when normal cells in your lungs change and grow out of control. It grows slowly compared to SCLC, but it can spread to other parts of the body.
Small Cell Lung Cancer (SCLC)
A rare fast-growing cancer that typically affects people who have a long history of tobacco use, specifically smoking cigarettes.
Stages of Lung Cancer (1-4)
This describes where the cancer is in the body and if it has spread outside the lungs.
Treatment
Whether or not you are currently on medication or have previously received treatment (approved or investigational) for your lung cancer.
Biomarker
The type of biomarker detected with your lung cancer. Cancer biomarkers are genes, proteins, or other substances that can be tested to reveal important details about a person's cancer.
Health
Your age and overall health, including current or past medical conditions. The study doctor will be able to go into more detail as this varies by clinical trial.
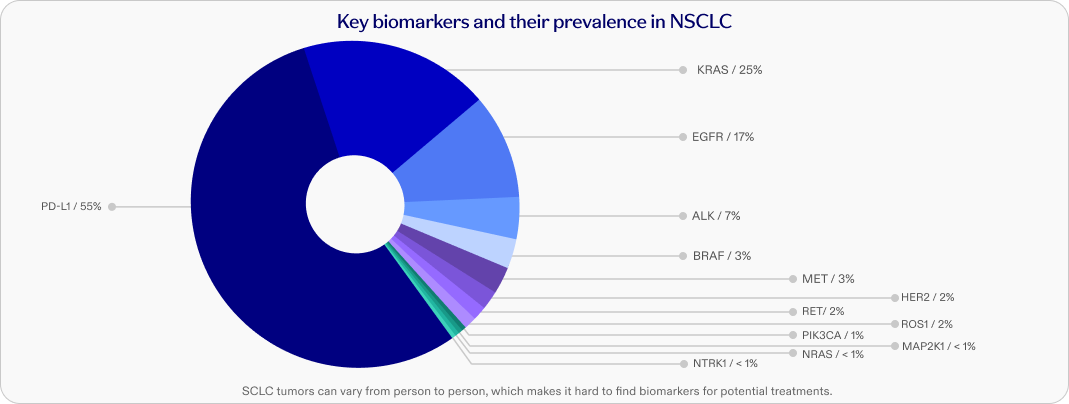
Biomarkers and targeted treatments
Healthcare providers test for biomarkers, often from a tumor tissue biopsy, to help guide treatment decisions. Pfizer is working to transform lung cancer treatment by researching new ways to approach the disease, such as studying potential treatments that target biomarkers.
Representation is key
Pfizer recognizes that to better understand health needs and the impact of our medicines, it is important that clinical trial participants represent the communities in which we conduct our trials and those disproportionately impacted by the diseases we aim to address. Many factors controlled by genetics, including race, ethnicity, and gender, can impact how people respond to a medicine and/or vaccine. So it's very important that clinical trials include people of all backgrounds.
- Overall, the chance that a man will develop lung cancer in his lifetime is about 1 in 17; for a woman the risk is about 1 in 181.
- Black men are about 12% more likely to develop lung cancer than White men. The rate is 16% lower in black women than in White women1.
- Asians and Pacific Islanders with lung cancer were 17% less likely to be diagnosed early compared to White people2.
- Latinx people with lung cancer are 30% more likely to not receive any treatment compared to White people2.
References

Frequently asked questions
In clinical trials, participant safety is the top priority. Before you participate, you will be given all the details about the trial, including potential benefits and risks of taking part. Your health will also be monitored while in the clinical trial.
If the study doctor determines that a trial is a good fit for you and you decide to take part, it's important to remember that this is your journey and choice. You are free to stop being in the trial at any time for any reason.
Depending on the design of the clinical trial, participants will generally either receive a study medicine, a standard-of-care treatment (the established treatment that is currently approved for people with a disease), or a combination of both. There is no placebo (inactive treatment) in a cancer clinical trial.
Pfizer generally covers the cost of the study medicine and all procedures that are part of our clinical trials. We are also committed to reimbursing participants for reasonable trial-related costs (e.g., travel, parking, meals). As each clinical trial is different, we encourage you to discuss this with the study staff.
Some potential benefits include:
- The research team will stay in touch and monitor your health during the trial.
- Participation may help people in the future by increasing our understanding of the study medicine.
Some potential risks include:
- All medical treatments (study or approved) may have side effects. Before joining any clinical trial, the staff will explain all the known potential side effects of the study medicine, any other treatments you will receive during your participation, and any potential risks from the study procedures (eg, the side effects of having a blood test).
- All known risks will be included in the Informed Consent Document (ICD) that you will have to sign before taking part.
The goals of a clinical trial are also called endpoints. These are results that researchers measure in a clinical trial to see how a study medicine affects people. The goals, or endpoints, vary study by study but may include:
- Whether the study medicine is safe
- Whether life expectancy is extended
- The percentage of patients who respond to the study medicine
- Whether there is an increase in the time that the cancer doesn't spread
- Whether there are changes to or improvements in quality of life
A description of each clinical trial and the results are available on ClinicalTrials.gov, as required by U.S. Law. You can search this website at any time.
If you choose to participate in a Pfizer clinical trial, we invite you to join other alumni by registering at www.PfizerClinicalTrialAlumni.com where, among other things, you will have access to news, resources, and general study results (also known as the Plain Language Study Results Summary) as soon as they become available. For some studies, participants may access a portion of their individual data.