Explore how enfortumab vedotin (EV)—an antibody-drug conjugate—is being studied alone and with other therapies.1-5
The safety and efficacy of these investigational compounds, or investigational uses of marketed products, have not been established. For an agent(s) whose safety and efficacy have not bewen established or confirmed, future regulatory approval or commercial availabiltiy is not guaranteed.
BCG=Bacillus Calmette-Guerin; EV=enfortumab vedotin; la/mUC=locally advanced or metastatic urothelial cancer; MIBC=muscle-invasive bladder cancer; MOA=mechanism of action; NMIBC=non-muscle-invasive bladder cancer.
Mechanism of action
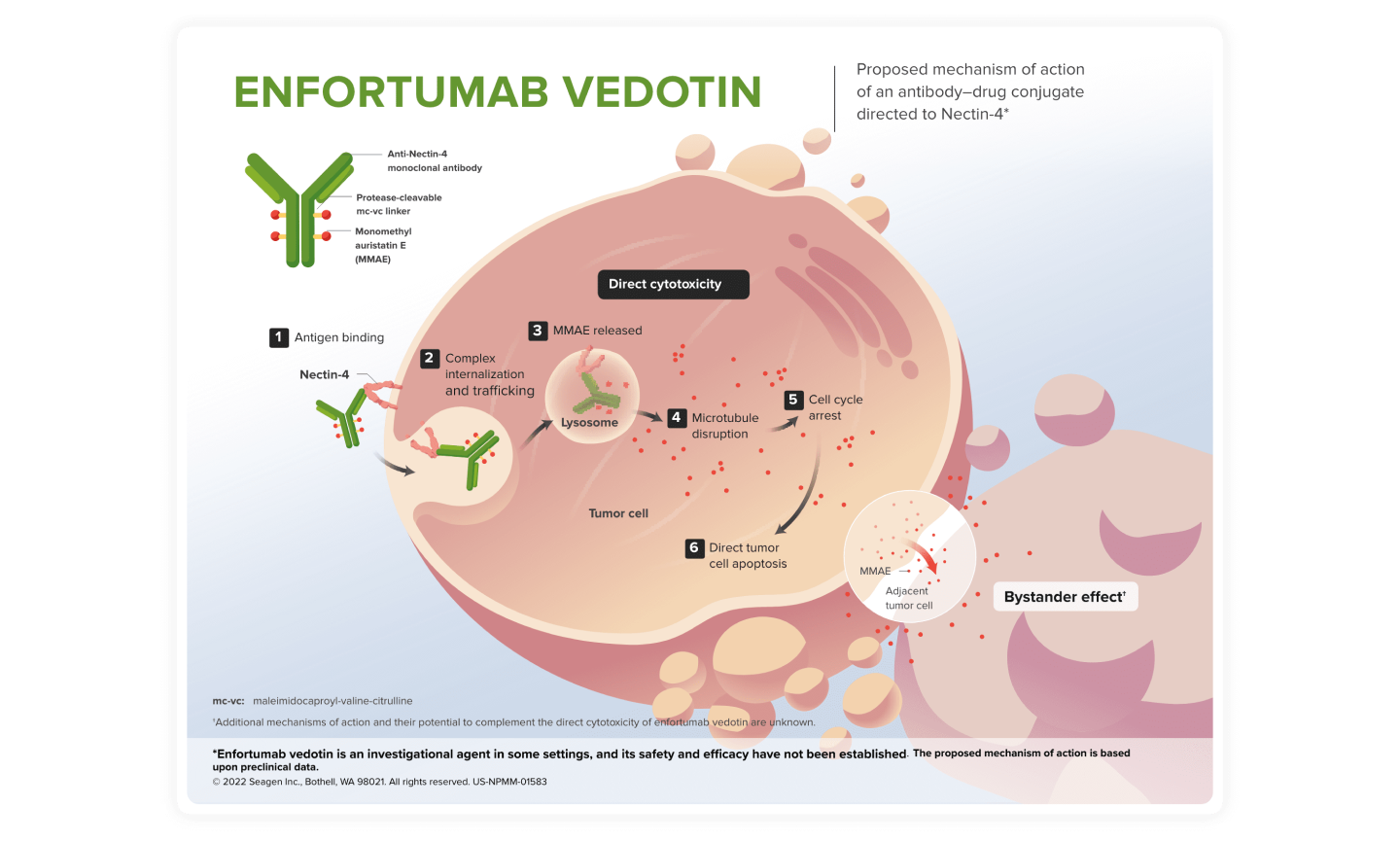
Proposed MOA of Enfortumab Vedotin (EV)
Enfortumab vedotin is an investigational agent in some settings, and its safety and efficacy have not been established.
Enfortumab vedotin:
An antibody-drug conjugate directed against Nectin-46,7
More to know
- EV is an antibody-drug conjugate (ADC) directed against Nectin-4 and is comprised of a fully human monoclonal antibody, the microtubule disrupting agent monomethyl auristatin E (MMAE), and a protease-cleavable linker. EV delivers MMAE to cells expressing Nectin-4, leading to cell cycle arrest and apoptotic cell death8
- Nectin-4 is a cell adhesion molecule that is expressed in both normal and tumor tissues. It is involved in multiple cellular processes known to be associated with oncogenesis, including cell adhesion, migration, proliferation, differentiation, and survival9,10
- High expression of Nectin-4 in select solid malignancies, including pancreatic, lung, breast, ovarian, and esophageal cancers, is associated with poor prognosis11-15
- The moderate to high expression levels of Nectin-4 in urothelial carcinoma (UC) and other tumors, compared with its low to moderate expression in normal tissues, make it a robust therapeutic target for drug development16
1. ClinicalTrials.gov. Perioperative pembrolizumab (MK-3475) plus cystectomy or perioperative pembrolizumab plus enfortumab vedotin plus cystectomy versus cystectomy alone in participants who are cisplatin-ineligible or decline cisplatin with muscle-invasive bladder cancer (MK-3474-905/KEYNOTE-905/EV-303) (07-14-2022). https://clinicaltrials.gov/ct2/show/nct03924895. Accessed 07-14-2022.
2. ClinicalTrials.gov. Perioperative enfortumab vedotin (EV) plus pembrolizumab (MK-3475) versus neoadjuvant chemotherapy for cisplatin-eligible muscle invasive bladder cancer (MIBC) (MK-3475-B15/KEYNOTE-B15/EV-304) (KEYNOTE-B15) (01-07-2021). https://clinicaltrials.gov/ct2/show/
3. ClinicalTrials.gov. A study to evaluate enfortumab vedotin in subjects with previously treated locally advanced or metastatic malignant solid tumors (EV-202) (01-13-2020). https://clinicaltrials.gov/ct2/show/nct04225117. Accessed 04-18-2022.
4. ClinicalTrials.gov. A study of intravesical enfortumab vedotin for treatment of patients with non-muscle invasive bladder cancer (NMIBC) (08-20-2021). https://clinicaltrials.gov/ct2/show/nct05014139. Accessed 04-18-2022.
5. Rosenberg J, O’Donnell PH, Balar AV, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol 2019;37(29):2592-600.
6. PADCEV [package insert]. Northbrook, IL: Astellas Pharma US, Inc.
7. Liu BA, Olson D, Snead K, et al. Additional mechanisms of action of Enfortumab vedotin, an anti-Nectin-4 ADC demonstrating bystander effect and immunogenic cell death antitumor activity in models of urothelial carcinoma. Poster presented at: AACR Virtual Annual Meeting II; June 22-24, 2020. https://www.aacr.org/meeting/aacr-annual-meeting-2020/
8. Rosenberg JE, O’Donnell PH, Balar AV, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol 2019;37(29):2592-600.
9. Boylan KLM, Buchanan PC, Manion RD, et al. The expression of Nectin-4 on the surface of ovarian cells alters their ability to adhere, migrate, aggregate, and proliferate. Oncotarget 2017;8(6):9717-38.
10. Zhang Y, Liu S, Wang L, et al. A novel PI3K/AKT signaling axis mediates nectin-4-induced gallbladder cancer cell proliferation, metastasis and tumor growth. Cancer Lett 2016;375(1):179-89.
11. Deng H, Shi H, Chen L, Zhou Y, Jiang J. Over-expression of Nectin-4 promotes progression of esophageal cancer and correlates with poor prognosis of the patients. Cancer Cell Int 2019;19(106):1-13.
12. DeRycke MS, Pambuccian SE, Gilks B, et al. Nectin 4 overexpression in ovarian cancer tissues and serum: potential role as a serum biomarker. Am J Clin Pathol 2010;134(5):835-45.
13. Nishiwada S, Sho M, Yasuda S, et al. Nectin-4 expression contributes to tumor proliferation, angiogenesis and patient prognosis in human pancreatic cancer. J Exp Clin Cancer Res 2015;34(30):1-9.
14. Takano A, Ishikawa N, Nishino R, et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res 2009;69(16):6694-703.
15. Zeindler J, Soysal SD, Piscuoglio S, et al. Nectin-4 expression is an independent prognostic biomarker and associated with better survival in triple-negative breast cancer. Front Med 2019;6(200):1-7.
16. Challita-Eid PM, Satpayev D, Yang P, et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res 2016;76(10):3003-13.