Disitamab Vedotin (DV) clinical trials
Examining disitamab vedotin (DV) alone or in combination with other therapies to provide target cancer treatments.
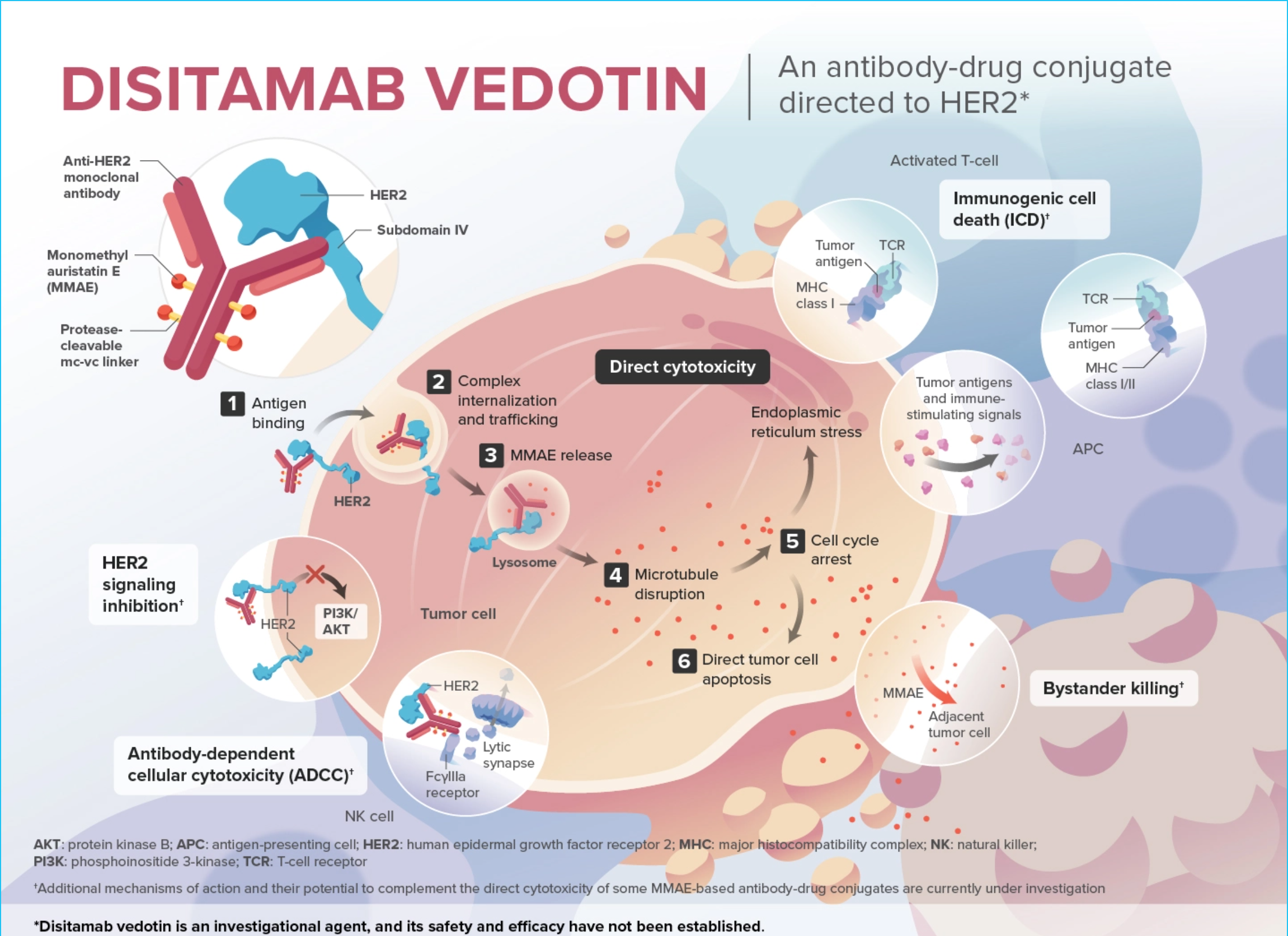
About Disitamab Vedotin
The study drug, disitamab vedotin (DV), is being developed for solid malignancies that express the human epidermal growth factor receptor 2 (HER2). DV is a novel, investigational antibody-drug conjugate (ADC), a clinically validated class of therapeutic compounds designed to target and selectively deliver chemotherapy into cancer cells through specific molecular targets expressed on tumors.
ADCs that target HER2 may induce cytotoxic killing through HER2 mediated delivery of cytotoxic payload. Additionally, through the bystander killing effect, ADCs demonstrate improvement in clinical activity beyond what has been seen with previous anti-HER2 agents. This allows HER2 receptors to remain targets for anti-HER2 therapies in HER2+ and HER2-low populations.
The safety and efficacy of these investigational compounds, or investigational uses of marketed products, have not been established. For an agent(s) whose safety and efficacy have not been established or confirmed, future regulatory approval or commercial availability is not guaranteed.
Disitamab vedotin is an investigational agent in some settings, and its safety and efficacy have not been established.
More to know
Antibody structure
- Disitamab vedotin (DV) contains three components: a recombinant, humanized, high infinity IgG1 monoclonal antibody directed to human epidermal growth factor receptor (HER2), a microtubule-disrupting agent monomethyl auristatin E (MMAE), and a protease-cleavable maleimidocaproyl-valine-citrulline (mc-vc) linker that covalently attaches MMAE to the antibody.
- DV binds to an distinct epitope on subdomain IV of the HER2 extracellular domain. 2,4
- DV is optimized for enhanced internalization and preferential release of cytotoxic MMAE within target cells.
Targeting HER2
- HER2 is a receptor tyrosine kinase2 that regulates cell proliferation and differentiation.3
- HER2 is overexpressed or amplified in multiple cancers, including breast, gastroesophageal, bladder, colon, head and neck, ovarian, endometrial, and lung.4
- HER2 is clinical validated as an oncogenic driver and/or therapeutic target in select cancers (breast, gastric, colon, and non-small cell lung cancer).7,8
References:
1. Jiang et al. 2016 Eu J Pharma, Jiang et al. 2020 Tox Letters, Li et al. 2016 CBT, Li et al. 2020 Eur Rev Pharm Sci, Yao et al. 2015 Breast Cancer Res, and RemeGen website.
2. Yao X, Cancer Res Treat. 2015: 122-33
3. Li H. Cancer Biol Ther. 2016: 346-54
4. Olayioye MA, Breast Cancer Res. 2001: 385-9
5. Jiang J. Eur J Pharm Sci. 2016 274-86
6. Li L. Eur Rev Med Pharmacol Sci. 2020: 12929-37
7. Iqbal N. Mol Biol Int. 2014: 852748
8. Strickler JH. ESMO GI 2022: Abstract LBA 2.