- Age: 18+ years
- Condition: Pathologically confirmed Stage IIIB or IIIC NSCLC and not a candidate for surgical resection or definitive chemoradiation, or Stage IV NSCLC
- Biomarker: Tumor has PD-L1 expression in ≥ 50% of tumor cells
- Documented negative test results for EGFR, ALK, and ROS1
Learn about a clinical trial for patients who have not been treated for PD-L1 high, Stage IIIb, Stage IIIc, or Stage IV NSCLC. Connect with a clinical trial site.
The Be6A LUNG-02 Clinical Trial is an open-label, randomized, Phase 3 study of sigvotatug vedotin, in combination with pembrolizumab, versus pembrolizumab monotherapy as first-line treatment in participants with PD-L1 high, locally advanced, unresectable, or metastatic non-small cell lung cancer (NSCLC).
Inclusion criteria
Inclusion criteria


Exclusion criteria


Exclusion criteria
Get started
Answer a 2-minute questionnaire and speak to a study representative.
A first step as you consider connecting with a Principal Investigator is to answer a 2-minute online questionnaire about your interest and willingness to be contacted. If your answers show the study might be a good fit for you and your patient, you may choose to have your contact information shared with a study clinic that you select for further discussion.
Get connected.
Your answers to these questions will only be linked to you if your responses indicate that you would like to be connected with a Principal Investigator and you choose to share your contact information with the study clinic. Pfizer study team members and our partners will have access to reports containing aggregated data that will not be directly linked back to you. Only the study staff can determine if your patient meets the study’s eligibility criteria and is able to enroll in the study.
About the Study Treatment
About Sigvotatug Vedotin
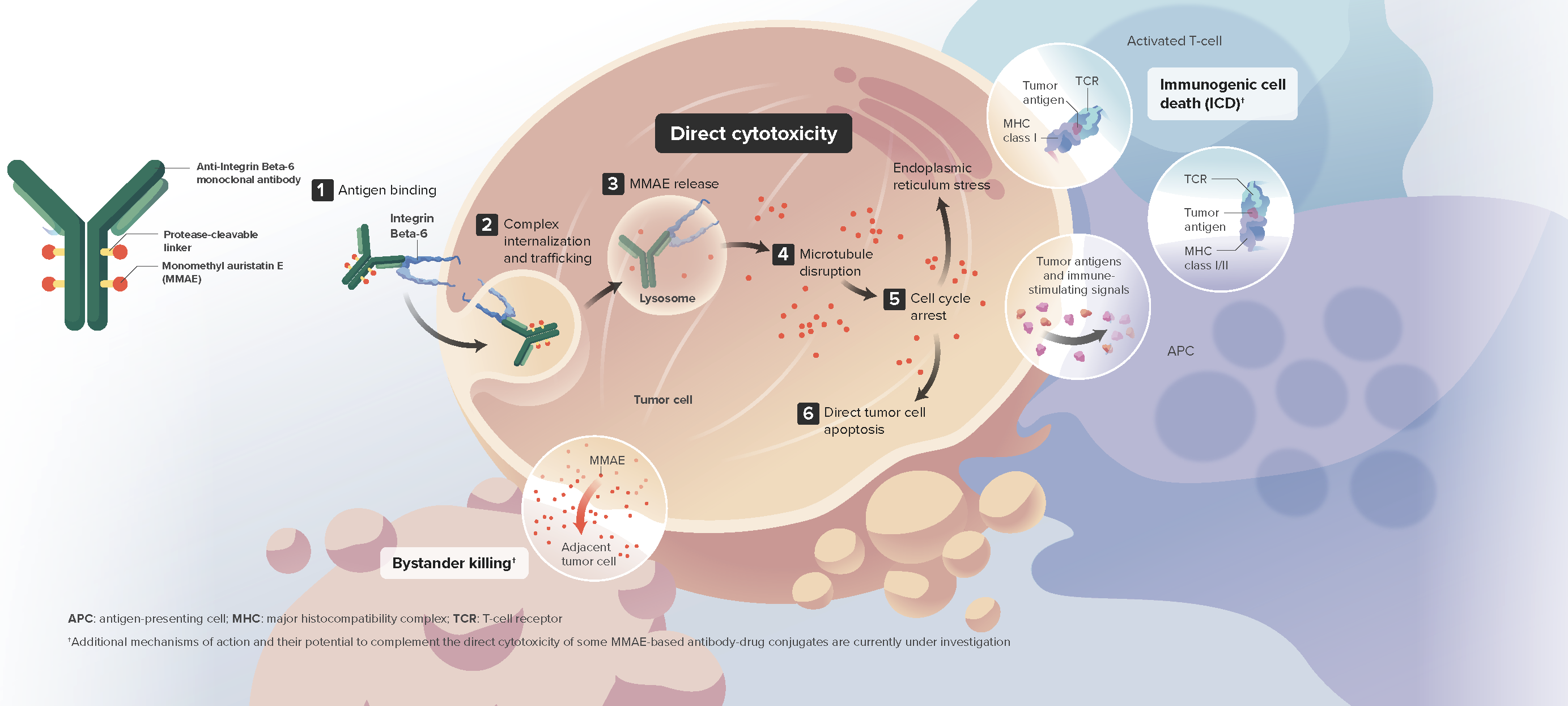
- Sigvotatug vedotin is an investigational antibody-drug conjugate (ADC) designed to deliver the cytotoxic payload monomethyl auristatin E (MMAE) to tumor cells expressing integrin beta-6 (IB6)1
- IB6 is a surface cell receptor overexpressed in multiple solid tumors such as non-small cell lung cancer, head and neck cancer, and esophageal cancer1,2
- Preliminary clinical evidence demonstrates antitumor activity and a manageable safety profile in untreated, locally advanced/metastatic NSCLC participants treated with sigvotatug vedotin plus pembrolizumab
Participants will be randomized 1:1 to receive either:
- Sigvotatug vedotin + pembrolizumab combination therapy
- Pembrolizumab monotherapy
Cycle length: 42 days
Sigvotatug vedotin IV Q2W (Days 1, 15, and 29)
Pembrolizumab IV Q6W (Day 1)
Primary Objectives
To demonstrate that sigvotatug vedotin + pembrolizumab is superior to pembrolizumab alone in prolonging:
- Overall survival
- Progression-free survival
Note: There are additional objectives in the protocol.
Length of Study Treatment
Up to 5 years
Length of study treatment is the length of time the study participants will receive the study treatment.

Number of Study Visits
Up to 5 visits in the first cycle, and 2 or 3 visits for all other cycles.
Each clinical trial’s design specifies the number of study visits and the total length of the trial.
Long-term Follow-up
Every 6 weeks for the first year and every 3 months afterwards. Follow-up visits may be done by phone, email, or in person.
Some studies require the study team to stay in contact with the participant for a period of time after the participant completes the main part of the study. This long-term follow-up is to collect additional information on the study drug over time.
What patients can expect
- Participants will receive pembrolizumab; some may also receive sigvotatug vedotin—there is no placebo
- All required activities happen on the same day as treatment (except for Cycle 1), minimizing the number of visits
- Ride assistance and travel/gas reimbursement are available
Proposed Mechanism of Action
Sigvotatug vedotin is thought to induce tumor cell death through:
- Direct cytotoxicity via preferential release of MMAE within target cells and subsequent apoptosis
- The bystander effect
- Immunogenic cell death3,4
About IB6-Overexpressing Cancers
IB6 is a member of the integrin family of proteins involved in cellular adhesion, motility, and cytokinesis. IB6 is expressed at low levels in normal adult epithelial tissues, but expression is induced by tissue injury due to its role in wound repair.5 High levels of IB6 expression have been demonstrated in different types of cancer1 and are associated with poor prognosis based on multiple retrospective analyses.6,7 Additional investigations suggest tumors may exploit the remodeling function associated with IB6 to promote invasiveness and metastasis.8,9
References
1. Lyon RP, Jonas M, Frantz C, et al. SGN-B6A: a new vedotin anti-drug conjugate directed to integrin beta-6 for multiple carcinoma indications. Mol Cancer Ther. 2023;22(12):1444-1453. doi:10.1158/1535-7163. MCT-22-0817
2. Brzozowska E, Deshmukh S. Integrin alpha v beta 6 (α v β6) and its implications in cancer treatment. Int J Mol Sci. 2022;23:(20):12346. doi:10.3390/ijms232012346
3. Lyon R, Trang V, Gosnik JJ, et al. Abstract 1522: SGN-B6A induces immunogenic cell death as an additional mechanism of action. J ImmunoTher Cancer. 2022; 10(suppl 2). doi:10.1136/jitc-2022-SITC2022. 1186
4. Trang VH, Mazahreh R, Gosnik JJ, et al. Abstract 1522: SGN-B6A induces immunogenic cell death as an additional mechanism of action. Cancer Res. 2023;83(suppl 7):1522-1522. doi:10.1158/1538-7445.am2023-1522
5. Van Aarsen LA, Leone DR, Ho S, et al. Antibody-mediated blockade of integrin alpha v beta 6 inhibits tumor progression in vivo by transforming growth factor-beta-regulated mechanism. CancerRes. 2008;68(2):561-570.doi:10.1158/008-5472.CAN07-0245
6. Elayadi AN, Samli KN, Prudkin L, et al. A peptide selected by biopanning identifies the integrin alphabeta6 as a prognostic biomarker for nonsmall cell lung cancer. Cancer Res. 2007:67(12):5889-5895.
7. Elez E, Kocakova I, Hokler T, et al. Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild-type metastatic colorectal cancer: the randomised phase I/II POSEIDON trial. Ann Oncol. 2015;26(1):13-140. doi:10.1093/annonc/mdu474
8. Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018:18(9):533-548. doi:10.1038/s41568-018-0038-z
9. Marsh D, Dickinson S, Neill GW, Marshall JF, Hart IR, Thomas GJ. Apha vbeta 6 integrin promotes the invasion of morphoeic basal cell carcinoma through stromal modulation. Cancer Res. 2008:68(9):3295-3303.doi:10.1158/0008-5472.CAN-08-0174