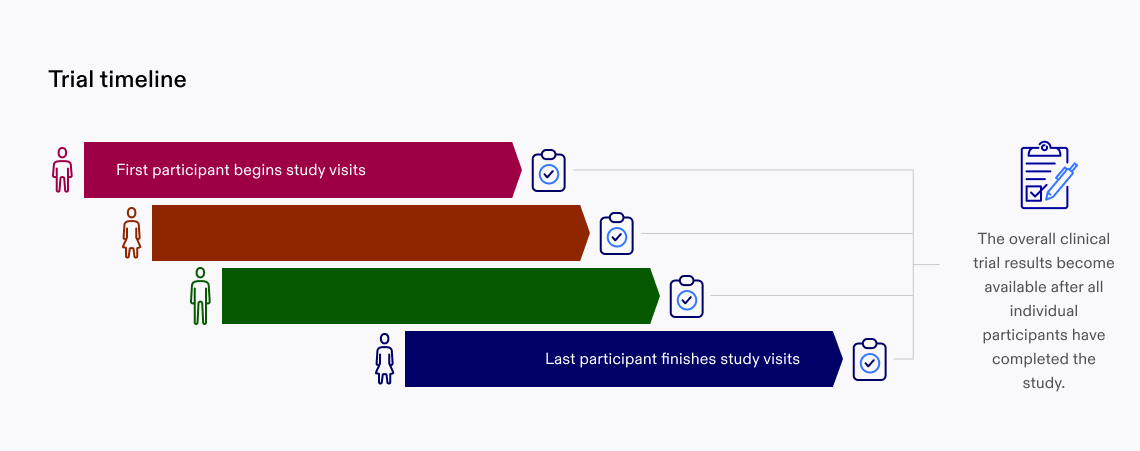
Now that you have completed your participation in the clinical trial, we still need to wait until the other participants have also completed their part. Once everyone completes the study, your clinical data, combined with everyone else’s, will tell us if the study was successful or not.
These results will help determine what happens next with the study medicine or vaccine. For example, we may continue to research the study medicine or vaccine in future clinical trials. If enough data from the trials support a safe and effective potential new medicine or vaccine, an application will then be submitted to regulatory authorities such as the FDA for review and potential approval for use by patients. This process often takes years.