Thank you for choosing to participate in a Pfizer clinical trial. We appreciate your strength and commitment because, without people like you, life-changing breakthroughs would not be possible. Now, we want to offer some information as you begin your clinical trial.
Because all clinical trials are different, how best to prepare for yours will depend on your study. Below are some things to consider and suggestions to keep in mind.
The four phases of clinical trials
1
As a participant in a phase 1 clinical trial, you’ll help researchers understand the safety of a study medicine. You may have frequent clinical exams and lab work, and will be asked to report any issues or side effects.
![]()
![]()
2
By joining a phase 2 clinical trial, you’re helping researchers better understand how well the study medicine may work for the condition being studied, and the side effects that may occur.
![]()

3
In a phase 3 clinical trial, you’ll be part of a larger group of people with the medical condition being studied. Your participation helps researchers determine whether the study medicine is safe and effective for people with that condition.


4
Even after medicines are approved for use, you can continue to participate in long-term clinical studies designed to better understand the effects of the approved medicine over time.

 Submission
Submission
 Review
Review
 Approval
Approval
 Available to Patients
Available to Patients
How clinical trials are designed
Protocol
Clinical trials start with a protocol. A clinical trial protocol is a detailed plan that explains the purpose of the clinical trial and how it will be run. It includes:

the length of the clinical trial

information about who can participate

the study medicines, procedures, and tests in the clinical trial

how side effects will be tracked, managed, and reported

the schedule of study activities

the rules that must be followed
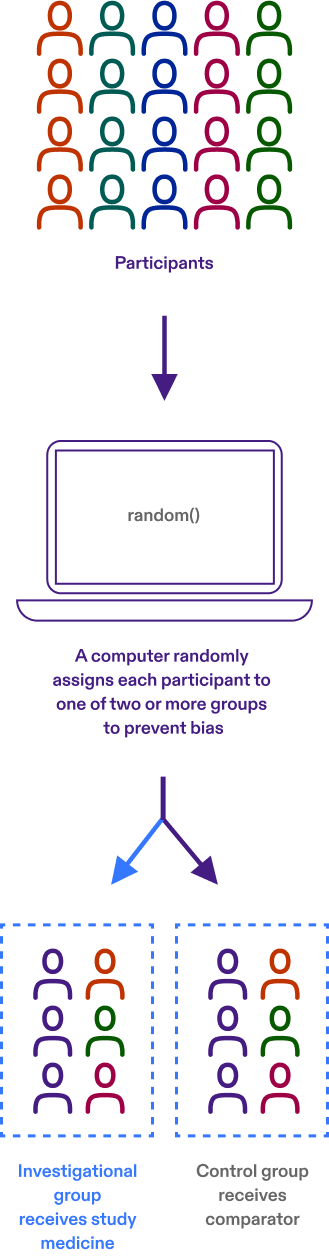
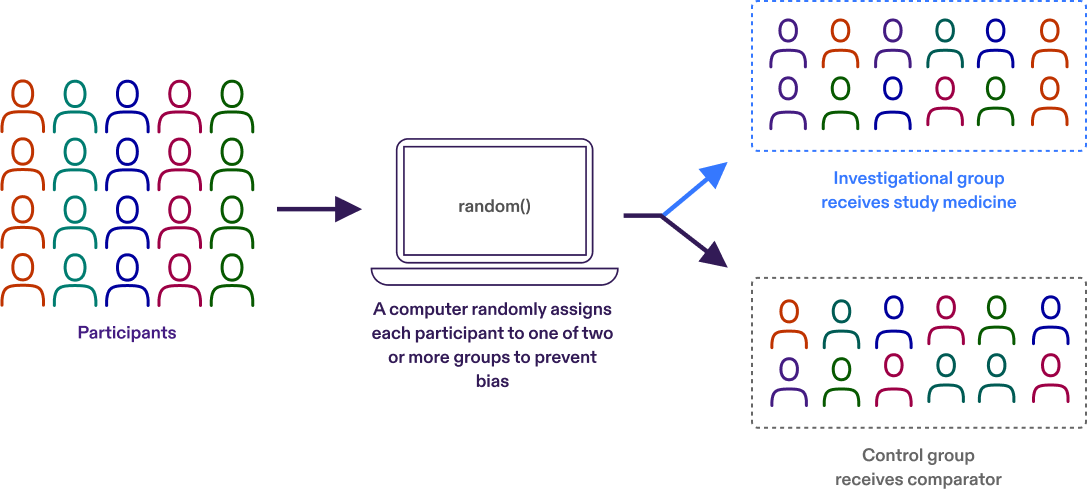
Randomization
Participants are assigned to different treatment groups in a clinical trial by a process called randomization. Randomization means that participants are assigned to a treatment group by chance (like flipping a coin) rather than by choice.
Randomization is one way to help avoid bias in a study. For example, it helps ensure that people of the same sex or age are not all assigned to the same treatment group.
Controlled trial
A controlled trial is a clinical trial that includes a comparison (control) group. In controlled clinical trials, participants are put into groups that receive the study medicine or a ‘comparator.’ We learn about the effectiveness and safety of the study medicine by comparing the experiences of the participants who receive the study medicine with those who receive the comparator.
A common example of a comparator is ‘standard of care,’ the established treatment that is currently used for a condition.
In a ‘double blind’ controlled trial, placebos are used to prevent the participant and study team from knowing whether the participant is receiving the study medicine or the comparator. A placebo does not contain any active ingredients, but the study medicine and placebo look alike. Learn more about single-blind and double-blind trials below.
Single-blind or double-blind clinical trials
Clinical trials could be single-blind or double-blind.
In a single-blind trial, the participants do not know whether they are receiving the study medicine or placebo, but the researchers know.
In double-blind trials, neither the participants nor the researchers know whether the participants are receiving the study medicine or placebo. (If necessary, such as for a safety reason, the researchers can find out what a participant has received.)
Clinical trials use blinding to help prevent bias. This way, the awareness of which treatment group a participant is in does not influence the participant or the study team.
All clinical trials are ‘unblinded,’ either after they are complete or when blinding is no longer necessary. When a study is unblinded, the assigned treatment group for each participant is revealed.